RABOME
Regenerative Arthritis & Bone Medicine, Inc.
Technology
Directing mesenchymal stem cells to bone with a novel compound: LLP2A-Alendronate
Osteonecrosis
RAB-001 as a treatment for osteonecrosis is an unmet medical need and is a viable candidate for development under the FDA Orphan Drug Guidelines. We estimate that RAB-001 could capture most of the available osteonecrosis drug sales in the US with sales in excess of $100 million+ per year. Given the patent and intellectual property portfolio for the drug, these sales could last 10+ or more years. Osteonecrosis started Phase I Human Clinical Trials by screening patients in November of 2016.Osteonecrosis (also called avascular necrosis, ischemic necrosis, and aseptic necrosis) is a disease caused by reduced blood flow to bones in the joints. As many as 20,000 people develop osteonecrosis each year in the United States; most are between ages 20 and 50. Orthopedists estimate that as many as ten percent of all primary total hip joint replacements performed in the United States are due to osteonecrosis. For reasons not fully understood, the incidence of osteonecrosis is much higher in other parts of the world; in China, Korea and Taiwan approximately one half of the primary total hip replacements are due to osteonecrosis. Some known causes of osteonecrosis are: steroid medications, alcohol use, injury, and increased pressure inside the bone. Risk factors for osteonecrosis are: radiation treatment, chemotherapy, kidney and other organ transplants.
Osteoporosis
Osteoporosis results from estrogen deficiencies and aging. It is the most common type of bone disease, and is expected to affect more than 61 million Americans by 2020. The disease occurs when the equilibrium between the formation of new bone and the reabsorption of old bone is disrupted in the body. In the U.S., 40 percent of women and 13 percent of men over the age of50 will suffer an osteoporosis-related fracture in their lifetimes, with resulting pain and limitations on activity. California has one of the nation's largest over-age-65 populations and can expect to experience greatly increased fracture rates because of the disease.Organize preclinical and clinical research to optimize the use of a small molecule (LLP2A-Ale) to direct endogenous mesenchymal stem cells to the bone surface for the treatment of osteoporosis. The novel approach has the potential to promote new bone growth and provide an effective answer for men and postmenopausal women and who suffer from a disease that causes increased bone fragility and often leads to bone fractures and a significantly decreased quality of life.
"The only currently approved therapy for osteoporosis that increases bone formation requires costly and inconvenient daily injections for two years," said Dr. Nancy Lane, our scientific consultant, who also serves as director of the UC Davis Center for Musculoskeletal Health. "I'm very optimistic that with our Disease Team grant from NIH and California Institute of Regenerative Medicine, we will be able to successfully, and fairly quickly, develop a bone-building treatment of osteoporosis that only requires an occasional infusion."
Rheumatoid Arthritis
Rheumatoid Arthritis causes joint swelling and pain and over time, functional impairment. Two million Americans suffer from rheumatoid arthritis (RA), an autoimmune disease that causes activation of inflammatory cytokines that destroy both cartilage and periarticular bone RA inflammation results in significant systemic and locale peri-articular bone loss that may due to activation of cytokines, such as tumor necrosis factor alpha (TNFα), interleukins and macrophage –colony stimulating factor (M-CSF) that increase osteoclast activity, the cells that break down bones, and inhibit osteoblast activities, the cells to make new bones. Bone loss associated with RA is further worsened by the use of the glucocorticoids (GCs), which remain frequently used for RA treatment. Although GCs are potent inhibitors of inflammation, GC use creates rapid bone loss that results in a high incident fracture risk. Despite the introduction of TNF alpha blocking agents that reduce inflammation and joint destruction, nearly 50% of RA patients treated in the US remain on GCs and 50% of RA patients treated with GCs develop fracture. Conventional drug therapies for RA mainly target inflammation but fall short of achieving the goal of treating bone loss. Antiresorptive treatment for bone loss such as bisphosphonates, and denosumab, are partially effective in reducing the number of new bone erosions. However bone-grow treatments to restore bone loss and improve bone strength are still needed.Bone regeneration through induction of Mesenchymal stem cells (MSCs) could promote osteogenesis and provide a rational therapeutic strategy for restoring bone loss induced by both the RA and medications used to treat RA. In addition, systemic MSC transplantations and homing of the MSCs to injury sites are shown to have anti-inflammatory effects as they express a variety of chemokine and cytokine receptors that help to reduce local inflammation. Accordingly, MSC transplantation offers an ideal treatment option for RA and RA induced bone loss through their potential anti-inflammatory and anabolic mechanisms.
However, previous attempts to use systematic MSC transplantation have failed to regenerate bone due to inability of the transplanted MSCs to home to bone except for some conditions that involve tissue injury repair such as fractures. Our compound, LLP2A-Ale, significantly increased homing of the transplanted MSCs to bone and the MSCs underwent osteoblast differentiation and prevented bone loss associated with estrogen deficiency or advanced aging. Our approach od using LLP2A-Ale overcomes the major obstacle in the transplantation of MSCs for bone regeneration. The combination treatment of LLP2A-Ale with MSC transplantation also represents a new therapy approach that not only treats bone loss but also may suppress inflammation associated with rheumatoid arthritis.
Fracture Healing
Traumatic fractures across the lifespan often require hospitalization, surgery, frequent physician visits, and lost time from work. More than 5 million Americans suffer from fractures annually. Each year an estimated 1.5 million individuals in the United States suffer a fracture just due to bone disease. In 2010, falls among older adults cost the US health care system $30 billion, and by 2020 it will be $68 billion in direct medical cost (Quoted from Center for Disease Control and Prevention). The morbidity associated with fracture healing is high and yet the effective treatment to accelerate fracture healing is still lacking. RAB-001 has very attractive potential as an aid in bone fracture healing with the potential to significantly accelerate the bone fracture healing time as well as reduce the associated inflammation involved with fracture healing. We estimate that RAB-001 could become a “stable” ingredient for bone fracture healing in the US in excess of $300 million+ per year. Given the patent and intellectual property portfolio for the drug, these sales could last 10+ or more years.
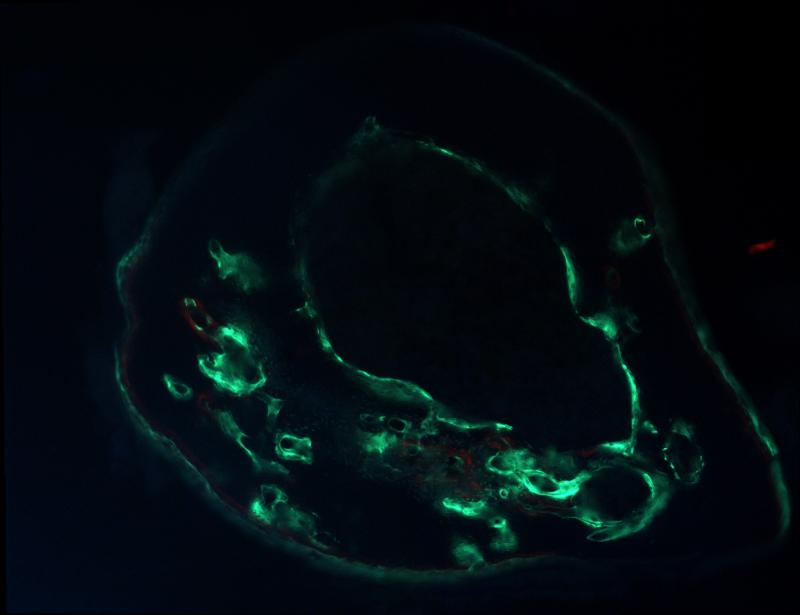
A mouse received LLP2A-Ale and mesenchymal stem cells treatment (MSCs). The MSCs were labeled with green-fluorescent protein that allowed visualization of the transplanted MSCs in bone.
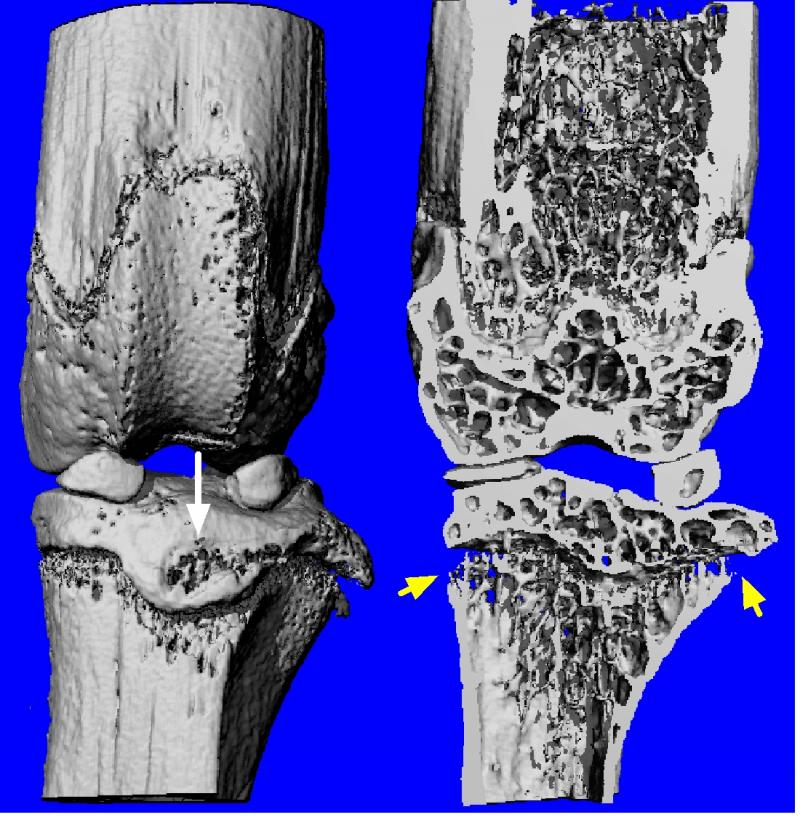
A mouse with RA before treatment, which showed were significant bone loss around the knee joint.
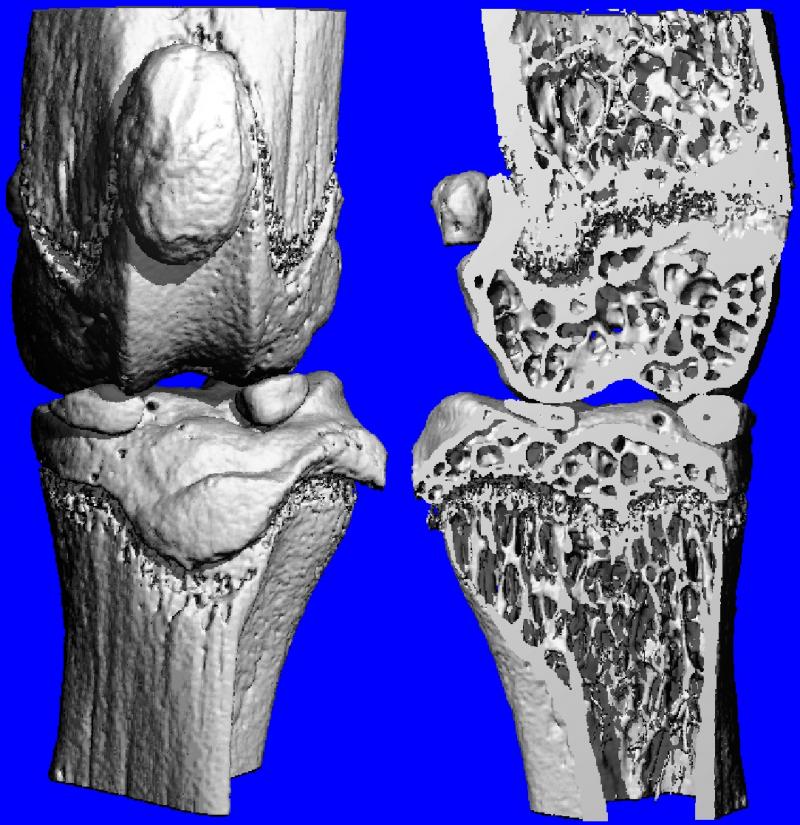 A mouse with RA that was treated with LLP2A-Ale, which showed no bone loss around the knee joint.
A mouse with RA that was treated with LLP2A-Ale, which showed no bone loss around the knee joint.
Copyright ©2013 RABOME. All rights reserved.